valence electron nitrogen|how to calculate valence electrons : Cebu Mar 23, 2023 Resultado da 13 de dez. de 2023 · Os Sacanas - Dormindo com Papai e Mamãe (primeira dublagem) : TUFOS : Free Download, Borrow, and Streaming : Internet .
0 · why is nitrogen triple bond
1 · valence electrons chart
2 · valence electron configuration for nitrogen
3 · nitrogen valence electrons lewis structure
4 · nitrogen valence electrons in neutral
5 · nitrogen valence electrons bonds
6 · nitrogen electron configuration full
7 · how to calculate valence electrons
8 · More
1 dia atrás · O resultado do concurso 5844 da Loteria Federal foi sorteado na noite de hoje, quarta-feira, 28 de fevereiro de 2024 (28/02/24). Prêmio de até R$ 500 mil. Os números .
valence electron nitrogen*******Mar 23, 2023 An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons .valence electron nitrogen Electron Configuration: 1s 2 2s 2 2p 3: Valence Electrons: 2, 5: Phase: Gas: . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of . Five. The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. Nitrogen has 5 electrons in its n=2 (outer) shell. There is a quick way of identifying the . Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of .
There are two ways to find the number of valence electrons in Nitrogen (N). The first is to use the Periodic Table to figure out how many electrons Nitrogen .
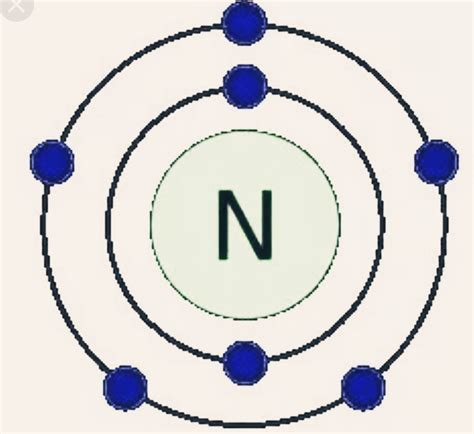
Electron Configuration: 1s 2 2s 2 2p 3: Valence Electrons: 2, 5: Phase: Gas: . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of .how to calculate valence electrons Electron Configuration: 1s 2 2s 2 2p 3: Valence Electrons: 2, 5: Phase: Gas: . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of .valence electron nitrogen how to calculate valence electrons In your case, nitrogen, N, is located in group 15, which means that it has 5 valence electrons. As you know, nitrogen exists as diatomic molecules, N2. Each nitrogen molecule consists of two atoms .
A. Nitrogen has 5 valence electrons and typically makes 5 bonds. B. Nitrogen has 5 valence electrons and typically makes 3 bonds. C. Nitrogen has 7 valence electrons and typically makes 1 bond. D. Nitrogen has 3 valence electrons and .

Nitrogen has 5 electrons in its outer shell and 2 electrons in its inner shell. Hence it has a total of 7 electrons. The valency of nitrogen depends upon which nitrogen ion is in question. An N+1 ion has a .In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can .Electron Configuration: 1s 2 2s 2 2p 3: Valence Electrons: 2, 5: Phase: Gas: . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total electrons in order to have a full valence shell, therefore it needs to have a triple .
These three bonds involve three of nitrogen’s valence electrons; the remaining two valence electrons occupy a non-bonding orbital and are referred to as a lone pair. . (2\mathrm{s}\) orbitals leading to four electron density centers around the nitrogen. The figure shows three representations of ammonia. The first indicates the \(\mathrm{N .A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. . In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen . Five. The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. Nitrogen has 5 electrons in its n=2 (outer) shell. There is a quick way of identifying the number of valence electrons - it is the same as the Group number (not for d-block elements, though). Nitrogen is in Group 5, so it has 5 .
In your case, nitrogen, N, is located in group 15, which means that it has 5 valence electrons. As you know, nitrogen exists as diatomic molecules, N2. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond. This is a direct consequence of the fact that each nitrogen atom has 5 valence .The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the . 9 years ago. The valence electrons of nitrogen in its compounds are all sp³ hybridized orbitals. The formal charge on N is usually -1 for an anion, 0 for a neutral compound, and +1 in cations. A nitrogen atom with a formal charge of -3 would correspond to a .A neutral nitrogen atom contains five valence electrons: 2s 2 2p 3. A nitrogen atom can therefore achieve an octet of valence electrons by sharing three pairs of electrons with another nitrogen atom. Because the covalent radius of a nitrogen atom is relatively small (only 0.070 nm), nitrogen atoms come close enough together to form very strong . Draw a valid electron dot structure for nitrogen. Solution. The elemental symbol for nitrogen is N. Based on Example \(\PageIndex{1}\) and Example \(\PageIndex{2}\), nitrogen has 5 valence electrons. Based on the rules described above, the first four dots must each be placed on their own "side" of the elemental symbol, and .Q: How many valence electrons does nitrogen have? How many valence electrons does nitrogen have? Flexi Says: Nitrogen has 7 electrons total, and 5 valence electrons. Nitrogen orbitals can hybridize, resulting in only 3 of the valence electrons available for bonding. Discuss further with Flexi. Ask your own question!
WEBDu kan vælge, hvordan du vil tjene penge – ved at spille spil, udfylde undersøgelser, se videoer, shoppe online og oprette konti. Samle overskud. Ressourcer. Ved at udføre .
valence electron nitrogen|how to calculate valence electrons